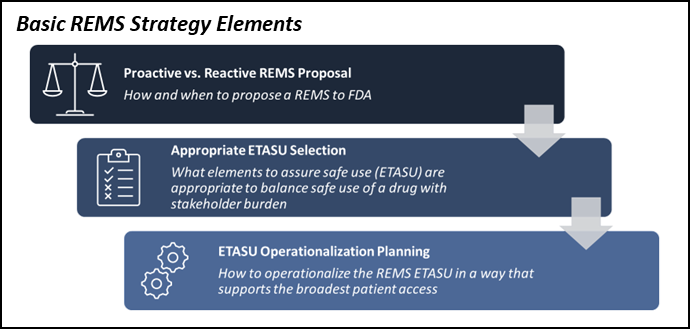
Avoid Launch Delays By Planning For An FDA-Required REMS Risk
Par un écrivain mystérieux
Last updated 03 juin 2024
lt;p>Picture this: The FDA accepts a manufacturer's NDA, and the manufacturer plans for its impending launch. But shortly before the anticipated approval, the FDA notifies the manufacturer that a Risk Evaluation and Mitigation Strategy (REMS) program is required to market the product. Now what?</p>
3 Components of US Medical-Device Regulation, Medical Devices and the Public's Health: The FDA 510(k) Clearance Process at 35 Years

White Paper, Missed Opportunities When Developing a REMS Program
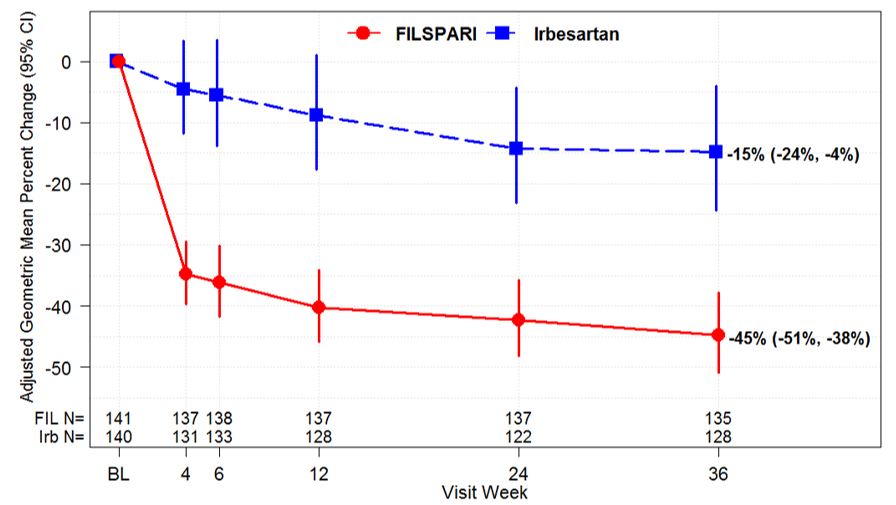
These highlights do not include all the information needed to use FILSPARITM safely and effectively. See full prescribing information for FILSPARITM. FILSPARITM (sparsentan) tablets, for oral useInitial U.S. Approval: 2023
PDF) Risk Evaluation and Mitigation Strategies (REMSs): Are They Improving Drug Safety? A Critical Review of REMSs Requiring Elements to Assure Safe Use (ETASU)

Disruptions Caused by iPLEDGE Modifications Are Negatively Impacting Patient Care - SDPA

FDA Regulation of Prescription Drugs
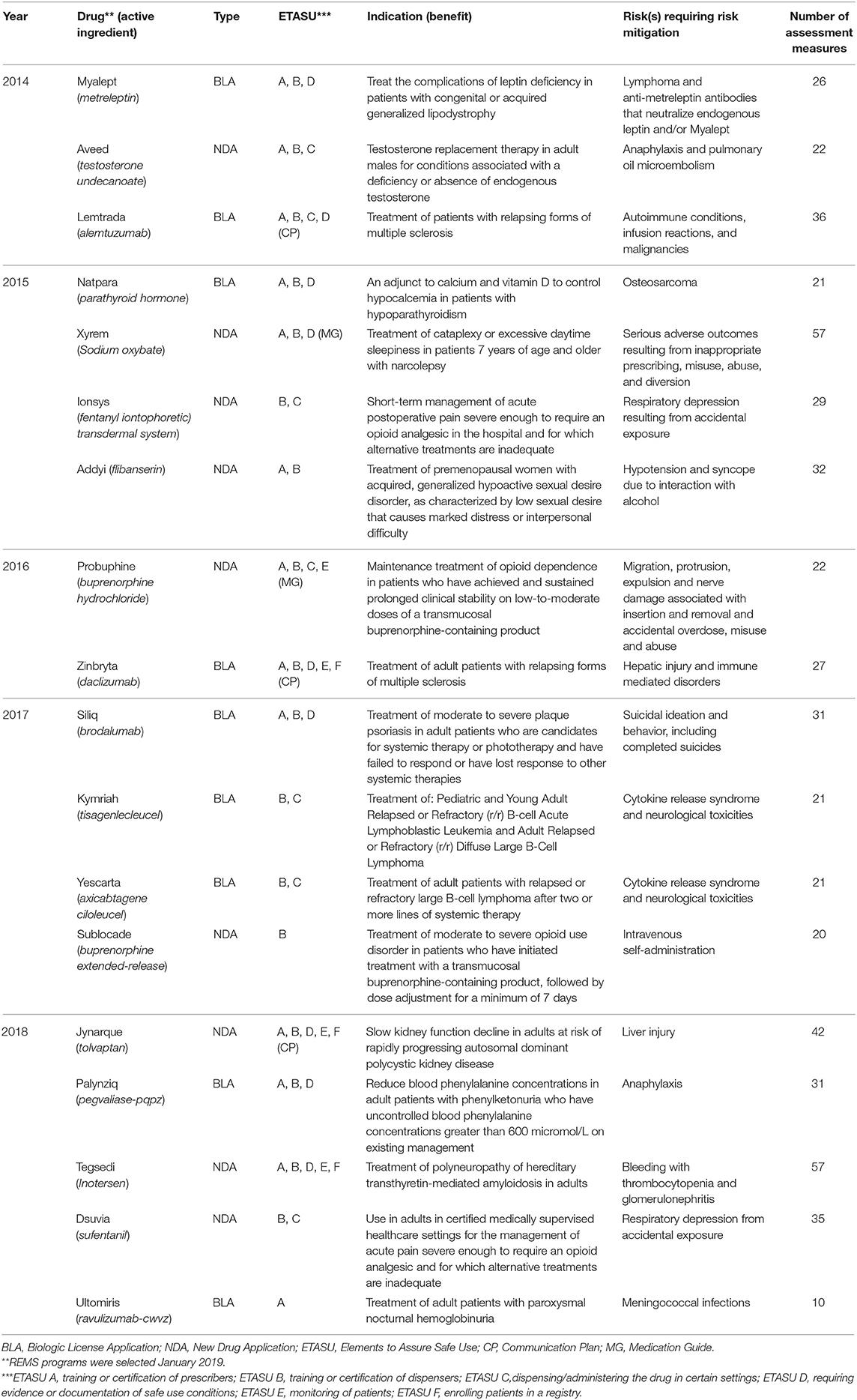
Frontiers Adaptation for Regulatory Application: A Content Analysis of FDA Risk Evaluation and Mitigation Strategies Assessment Plans (2014–2018) Using RE-AIM

Drug Safety and the Cost of Monitoring: The Role of REMS in Risk Management - Mark Slomiany, Rema Bitar, Sarah Kruse, Sarah Jeffers, Kenneth Berkowitz, Mahmud Hassan, 2015

Ensuring FDA Regulatory Success for Biomedical Companies -- Key Lessons for Start-Ups

Flawed safety rules limit a highly effective schizophrenia treatment - STAT
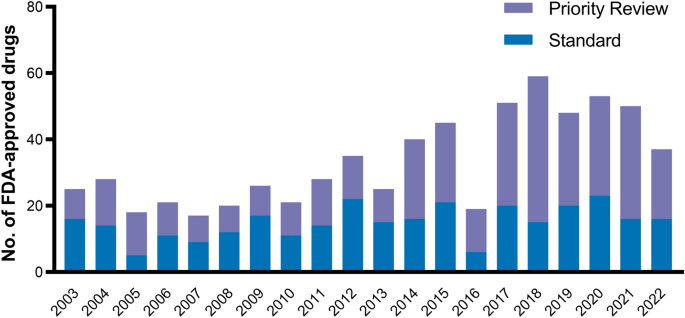
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy

Avoid Launch Delays By Planning For An FDA-Required REMS Risk Evaluation and Mitigation Strategy
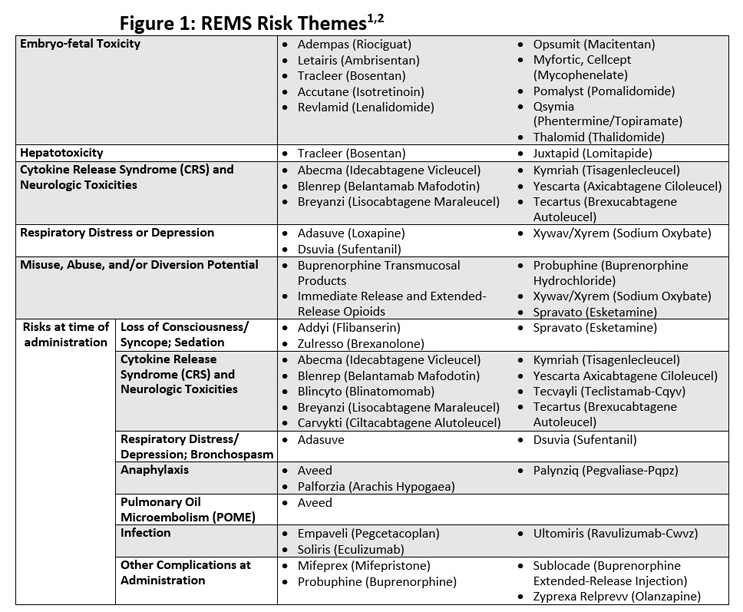
Avoid Launch Delays By Planning For An FDA-Required REMS Risk Evaluation and Mitigation Strategy
Recommandé pour vous
 REMS - Akku-Curvo 22v Cordless Pipe Bender - No Formers Included - (580014)14 Jul 2023
REMS - Akku-Curvo 22v Cordless Pipe Bender - No Formers Included - (580014)14 Jul 2023 REMS Mini-Press S 22V ACC - Sertisseuse radiale électro14 Jul 2023
REMS Mini-Press S 22V ACC - Sertisseuse radiale électro14 Jul 2023 Presse à tubes REMS Power-Press SE - Toru-Jüri14 Jul 2023
Presse à tubes REMS Power-Press SE - Toru-Jüri14 Jul 2023 Pince à sertir (Mâchoire) REMS profil H Ø2614 Jul 2023
Pince à sertir (Mâchoire) REMS profil H Ø2614 Jul 2023- Rems : Produits à prix professionnels - - Warmango14 Jul 2023
 Pince à sertir MINI-PRESS REMS 22V ACC14 Jul 2023
Pince à sertir MINI-PRESS REMS 22V ACC14 Jul 2023 REMS Cento ou Cento RF - Coupe-tube électrique à molette - Tuyaux14 Jul 2023
REMS Cento ou Cento RF - Coupe-tube électrique à molette - Tuyaux14 Jul 2023 REMS : produits REMS commercialisés par Les Matériaux14 Jul 2023
REMS : produits REMS commercialisés par Les Matériaux14 Jul 2023 REMS for Scientists REMS – NASA Mars Exploration14 Jul 2023
REMS for Scientists REMS – NASA Mars Exploration14 Jul 2023 REMS Mini Press 22v14 Jul 2023
REMS Mini Press 22v14 Jul 2023
Tu pourrais aussi aimer
 Kit Biscuit de Noël Sapin 8,2 cm Scrapcooking : achat, vente14 Jul 2023
Kit Biscuit de Noël Sapin 8,2 cm Scrapcooking : achat, vente14 Jul 2023 Champions League Cup Vector Art Champions league trophy, Champions league, Liverpool champions league14 Jul 2023
Champions League Cup Vector Art Champions league trophy, Champions league, Liverpool champions league14 Jul 2023 Cuillère Doseuse à Café 10g - KINTO14 Jul 2023
Cuillère Doseuse à Café 10g - KINTO14 Jul 2023 A Fun and Challenging Quordle Puzzle: September 2114 Jul 2023
A Fun and Challenging Quordle Puzzle: September 2114 Jul 2023 GLASS™ non-slip self-adhesive, transparent mat,protection for wooden stairs MIX14 Jul 2023
GLASS™ non-slip self-adhesive, transparent mat,protection for wooden stairs MIX14 Jul 2023 CALVENDO Technologie . Avions des patrouilles de Sudpastel Sudpastel - Livre - Decitre14 Jul 2023
CALVENDO Technologie . Avions des patrouilles de Sudpastel Sudpastel - Livre - Decitre14 Jul 2023 Bonnet Odlo Adulte POLYKNIT WARM ECO14 Jul 2023
Bonnet Odlo Adulte POLYKNIT WARM ECO14 Jul 2023 POSCA Pochette de 4 marqueurs POSCA. Pointe fine conique 0,9-1,3mm. Coloris : or, argent, noir, blanc14 Jul 2023
POSCA Pochette de 4 marqueurs POSCA. Pointe fine conique 0,9-1,3mm. Coloris : or, argent, noir, blanc14 Jul 2023 Chauffage électrique en gros, ventilateur de chauffage en céramique ptc sûr et silencieux14 Jul 2023
Chauffage électrique en gros, ventilateur de chauffage en céramique ptc sûr et silencieux14 Jul 2023 Damart - Manches longues, col rond - Femme - Grijs - M14 Jul 2023
Damart - Manches longues, col rond - Femme - Grijs - M14 Jul 2023
